Spectrum of Science 4.20
The world is colorful for us people. But how do colors come about? Here we present surprising experiments on color mixing, some of which can also be done at home.
When children have a watercolor box in front of them for the first time, they do not yet know what will come out when they combine the different colors. Therefore, the first experiment is perhaps more exciting for little observers than for adults: mix the yellow and blue colors of a watercolor box. The fact that you get green should hardly surprise anyone.
The second experiment is a little more complex and less predictable. You need the following “ingredients”:
- Red ink from the company Lamy
- Yellow text marker from either Herlitz, Pelikan or Faber-Castell as they contain the fluorescent dye pyranine
- Methylated spirits (ethanol)
- Two glass containers for more than 10 ml (e.g. 0.2-cl shot glasses)
- 2 x 8 cm strip of a plastic film, such as colorless, thin packaging film
- Drip pipette
Step 1:
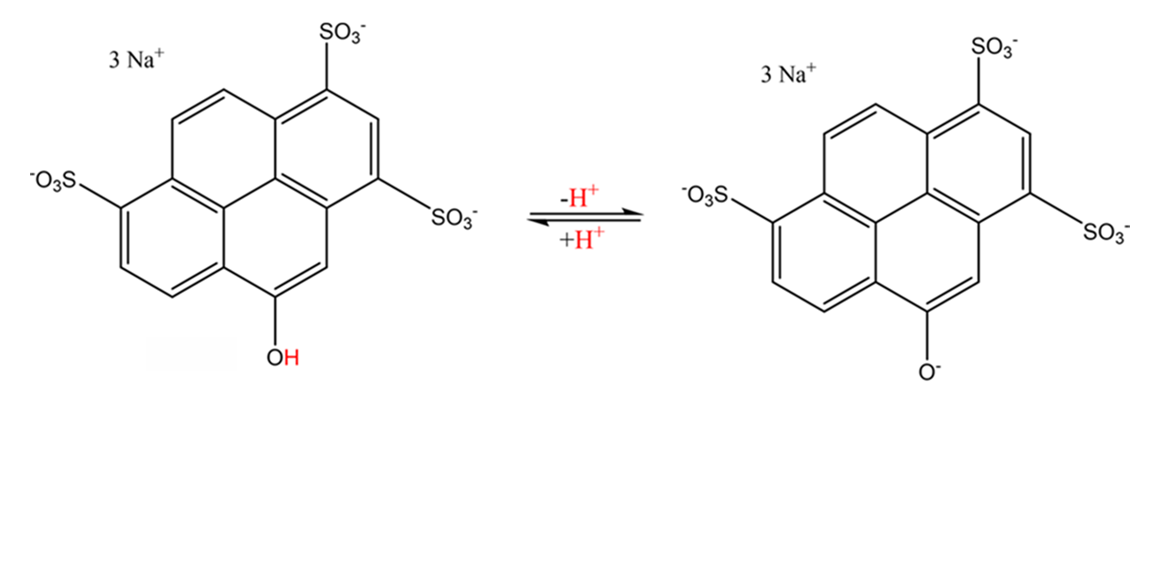
Using a suitable measuring cup, fill both glasses with 10 ml of methylated spirits. The foil strip is painted on an area of three by two centimeters with the yellow text marker and dipped into one of the jars. It is then removed, dried with a household cloth and the entire process is repeated twice with the same solution. Thus, the yellow text marker paint dissolves in methylated spirits. At first you will not notice it, because the resulting solution remains colorless. The reason for this is the special properties of pyranine, which in solution forms an equilibrium between a protonated, colorless form and a deprotonated, yellow form (structural formulae above). In a neutral, aqueous environment, the state of equilibrium is so far on the right side of the equation that a pyranine solution appears yellow: Pyranine molecule ions readily release a proton (H+) of their OH group to water, there are so many deprotonated pyranine particles that the human eye can perceive the yellow color. In ethanol, a colorless solution is obtained, since ethanol tends to absorb protons far less than water, and the equilibrium is therefore almost completely on the left (colorless) side.
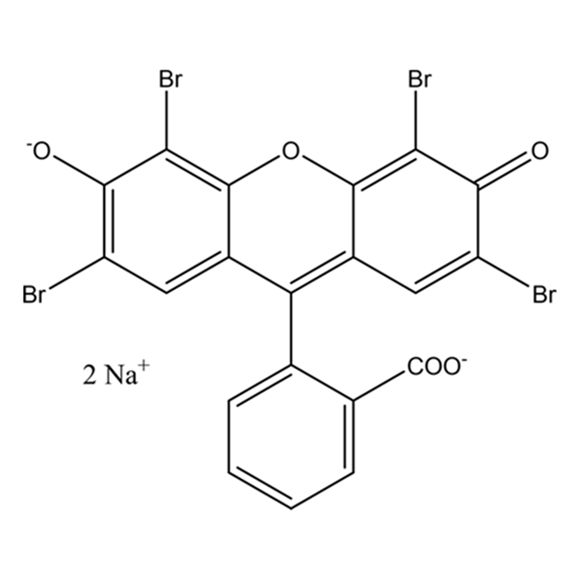
Figure 2: The colorant eosin Y is contained in some red inks
Step 2:
Add a drop of red Lamy ink to the ten milliliters of ethanol in the second shot glass. This causes the solution to change to a very faint red color and it fluoresces slightly yellow. This is because the ink contains the colorant eosin Y.
The solutions produced provide an impressive picture under UV light (wavelength 365 nm), as the pyranine solution fluoresces intensively blue and the eosin Y solution glows yellow
© Matthias Ducci/ Pädagogische Hochschule Karlsruhe


Step 3:
Like the yellow and blue watercolor, the yellow and blue solutions are now mixed in another vessel under UV light. We asked many students and pupils beforehand what they would expect. The overwhelming majority expected to receive a green, fluorescent solution. Be honest, what did you think?
In fact, the mixture of both solutions fluoresces in white.
© Matthias Ducci/ Pädagogische Hochschule Karlsruhe
Subtractive versus Additive Color Mixing
The false expectation of the pupils and students can be explained by the fact that they applied the principle of subtractive color mixing to the present experiment. They have firmly internalized this principle, since they have known it - most likely not under this name - since elementary school from mixing watercolors.
In case of subtractive color mixing, part of the light is blocked by color filters or absorbed by pigments and thus, subtracted from the original light. The remaining light forms a mixed color. Thus, subtractive color mixing always occurs when bodies that do not glow themselves create a color impression. Already the three basic colors cyan, magenta and yellow are sufficient to mix all imaginable colors.
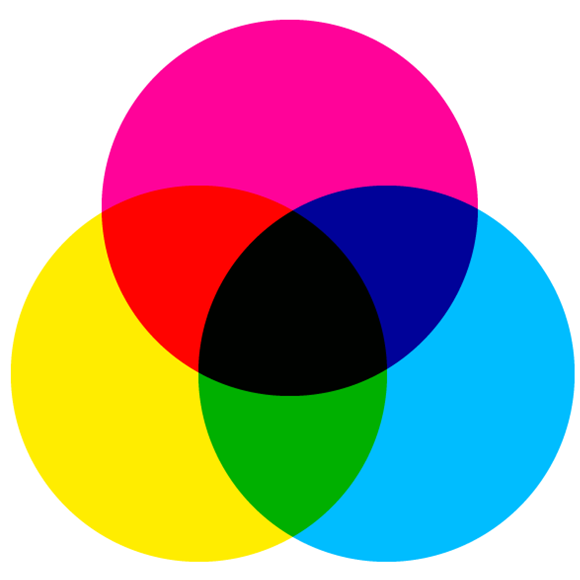

In contrast, the experiment with the fluorescent solutions results in additive color mixing. This always happens when colored light from different light sources meet. The resulting color impression depends on the original colors and their intensity. The three basic colors are red, green and blue-violet (RGB). According to international standards, this is monochromatic light with wavelengths of 700 nanometers (nm) (red), 546 nm (green) and 435 nm (blue-violet). If these are added in suitable brightness, one will see the color white. A mixture of green and red produces yellow, green and blue-violet produce cyan, and blue-violet and red produce magenta. Other (mixed) colors can be created by changing the light intensity.
References:
[1] Ducci, M., Oetken, M.: Wie Farben entstehen. Spektrum der Wissenschaft 4/2020, S. 48-51. Publication approved by Spektrum der Wissenschaft Verlagsgesellschaft mbH, Heidelberg 2020
[2] Ducci, M., Oetken, M.: Fluoreszierende Farbspiele. Spektrum.de 21.02.2018. Publication approved by Spektrum der Wissenschaft Verlagsgesellschaft mbH, Heidelberg 2020